Abstract
Introduction: Pediatric non-Hodgkin lymphomas (NHLs) comprise approximately 10% of all childhood cancers. The diagnosis requires extensive workup with multiple ancillary studies. Genetic testing using fluorescence in situ hybridization (FISH) is a cytogenetic approach that is often utilized in pediatric NHL.
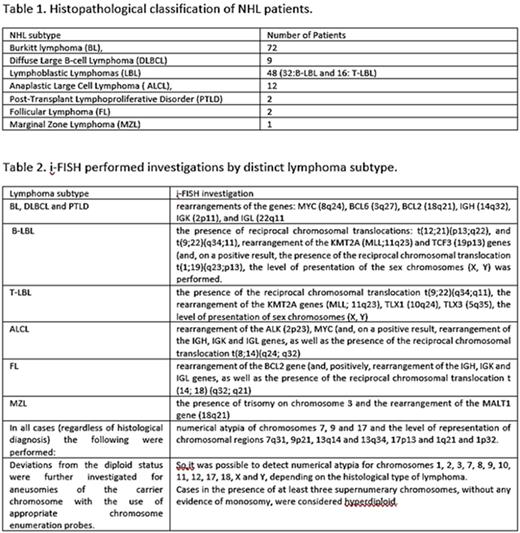
Patients and Methods: Our study included 146 children (113 boys-33 girls), aged 0.33-17.8 years (median 9.16 years), with NHL diagnosis based on histopathology (table 1). The histological diagnosis was based on the complete microscopic and immunohistochemical study of infiltrated tissue. Touch imprints from the diagnostic biopsy samples were further investigated with i-FISH (table 2).
Results: In all BL cases MYC gene rearrangement was identified. At least one additional chromosomal aberration was also found in 47 patients. Six hyperdiploid cases were identified among 72 children (8.3%), with at least 3 chromosomes involved. Interestingly, there were no cases of refractory disease, relapse, or death among hyperdiploid patients, and their overall survival (OS) was comparable to that of MYC rearrangement subgroup, a unique finding. Among LBL patients, a high frequency of 9p21 region deficiency was found, as in Acute Lymphoblastic Leukemia (ALL). Also, the chromosomes most frequently involved in hyperdiploid B-LBL (X,17,21) were identical to chromosomes commonly overrepresented in ALL. In contrast, the absence of cases positive for ETV6/RUNX1 hybrid gene is surprising. Possibly, the incidence of the most common cytogenetic atypias is different between leukemia and lymphoma. Of note, the two patients with KMT2A rearrangement achieved complete remission after first-line therapy. Finally, we identified cases with a normal karyotype but existing cytogenetic atypia demonstrable by i-FISH exclusively in LBLs. ALK gene rearrangement was found in all ALCL patients while no MYC gene rearrangement was found. At least one additional atypia was identified in 6/12 patients. Interestingly, ALCL patients had proportionally more recurrences than any other histological category, but without a corresponding increase in mortality. Among DLBCL group we found the highest rate of hyperdiploidy (22.2%). Only two (non-hyperdiploid) patients with BCL6 gene rearrangement were found. The single patient who relapsed had an IGL gene rearrangement, suggesting activation of an oncogene in the context of reciprocal chromosomal translocation involving the 22q11 region. No MYC or BCL2 gene rearrangements were found among DLBCL cases. In hyperdiploid patients, overrepresentation of chromosome 8 was found, along with overrepresentation of chromosome 3 (and, in addition, 18 in one of the two cases). This combination of atypias apparently leads to supernumerary copies of MYC gene, simultaneously with the presence of supernumerary alleles of BCL6 (or BCL2, respectively). Regarding findings in FL, MZL and PTLD, due to the small number of patients in these subgroups, any observation is of limited value. Absence of BCL2 gene rearrangement in childhood FL was confirmed. In the study's only patient with MZL, the identification of trisomy 3 may not be accidental, since this atypia is common in the corresponding adult disease. Of particular interest is the one patient with PTLD. Although monomorphic Burkitt-type PTLD was diagnosed and B-clonality documented based on VDJ recombination of IGH gene, marrow karyotyping and thorough i-FISH screening revealed no chromosomal atypia. The phenomenon has been highlighted in the literature and indicates that PTLD's aggressive clinical course is not necessarily associated with genomic instability and damage. In our study no patient met double-hit lymphoma criteria. We might wonder whether, in our series, the hyperdiploid patients with supernumerary copies of intact MYC, BCL2 and/or BCL6 genes constitute a subgroup of the "alternative" double hit lymphomas. With a median follow-up time of 61.5 months (range 7-123 months), 20 patients succumbed, yielding: 3-year and 5-year progression free survival (PFS) in all patients 84.5% και 82.5% respectively, and 3-year and 5-year OS in all patients 86.7%.
Conclusions: This extensive i-FISH investigation provides clinically meaningful information on the genetic profile of NHLs, raises new questions, and points to directions for further investigation within the field of childhood lymphoproliferation.
Disclosures
Kattamis:IONIS: Consultancy; AGIOS Pharmaceuticals: Consultancy; Novartis: Consultancy, Honoraria, Research Funding; ViFOR: Consultancy; AMGEN: Consultancy; VERTEX: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria, Research Funding; Chiesi: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal